Read the most recent review: 338, 357, 364, 388, 396, 412, 414
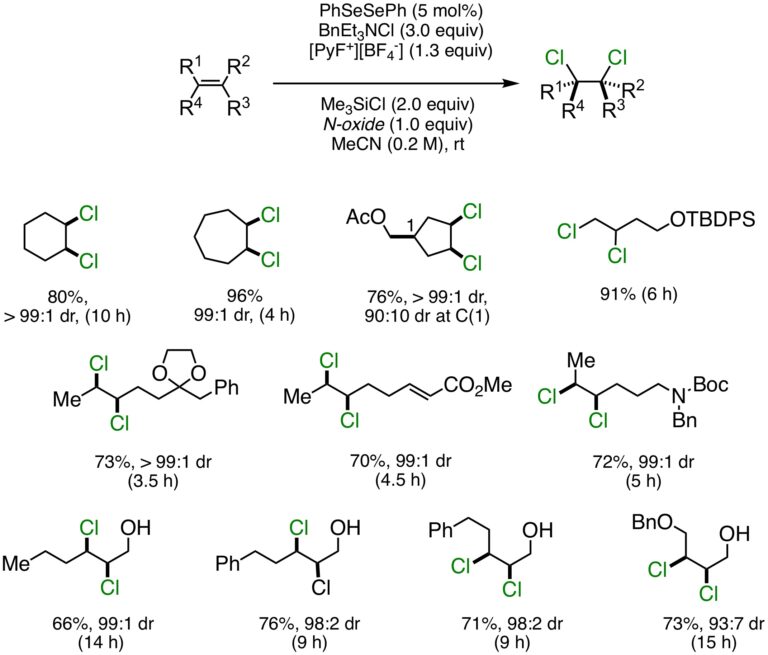
As some of the oldest organic chemical reactions known, the ionic additions of elemental halogens such as bromine and chlorine to alkenes are prototypical examples of stereospecific reactions, typically delivering vicinal dihalides resulting from anti-addition. Whilst the invention of enantioselective variants is an ongoing challenge, the ability to overturn the intrinsic anti-diastereospecificity of these transformations is also a largely unsolved problem. We have developed the first catalytic, syn-stereospecific dichlorination of alkenes, employing a group transfer catalyst based on a redox-active main group element (i.e., selenium). Thus, with diphenyl diselenide (PhSeSePh) (5 mol %) as the pre-catalyst, benzyltriethylammonium chloride (BnEt3NCl) as the chloride source, and an N-fluoropyridinium salt as the oxidant, a wide variety of functionalized cyclic and acyclic 1,2-disubstituted alkenes, including simple allylic alcohols, deliver syn-dichlorides with exquisite stereocontrol. This method is expected to find applications in streamlining the synthesis of polychlorinated natural products.
Catalytic, Stereospecific, Syn-Dichlorination: Scope:
Catalytic, Stereospecific, Syn-Dichlorination: Catalytic Cycle:
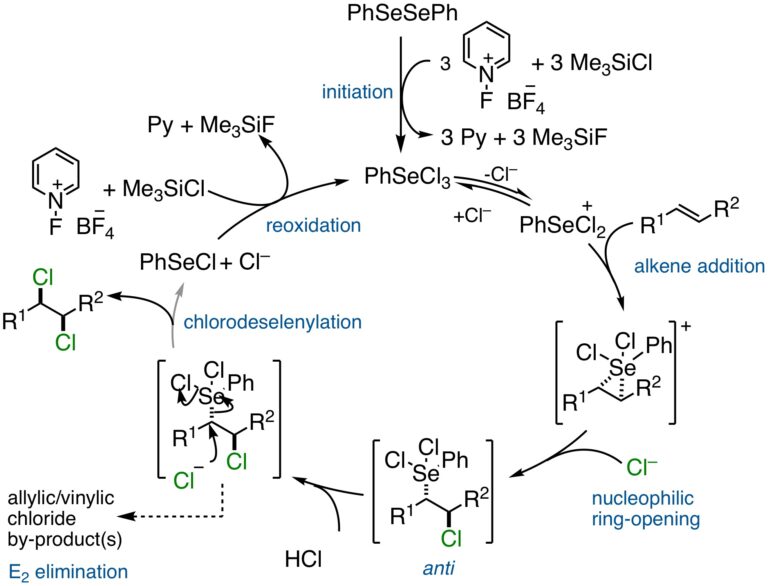
Elevating the syn-stereospecific dichlorination to an enantioselective reaction encountered unexpected challenges. Despite a heroic effort in the synthesis and evaluation of scores of chiral, selenium-based catalysts, the enantioselectivities plateaued at a modest level (ca. 75:25 er). Careful mechanistic experiments revealed that the likely cause was the reversibility of the initial chloroselenylation owing to the slow, second displacement by the weakly nucleophilic chloride ion.
Accordingly, a potential solution would be to increase the nucleophilicity of the addends and tether the two nucleophilic groups to accelerate the second displacement to capture the intrinsic face selectivity of the catalytically active selenium species. This strategy proved successful in the employment of a urea bifunctional nucleophile using the most selective diselenide catalyst and otherwise identical conditions.
Catalytic, Enantioselective, Syn-Diamination: Scope:
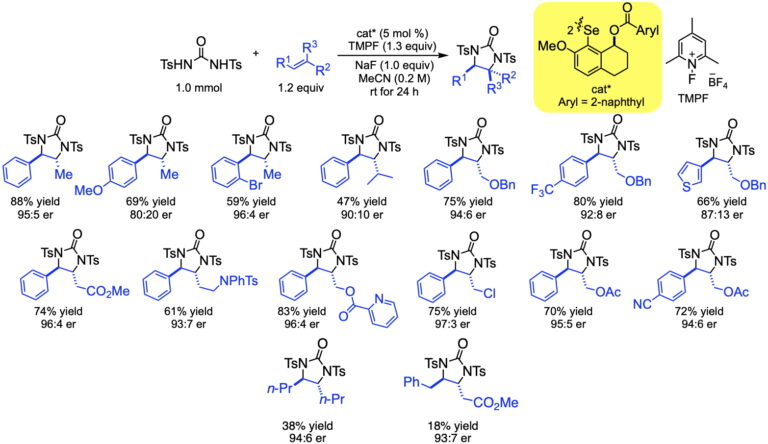
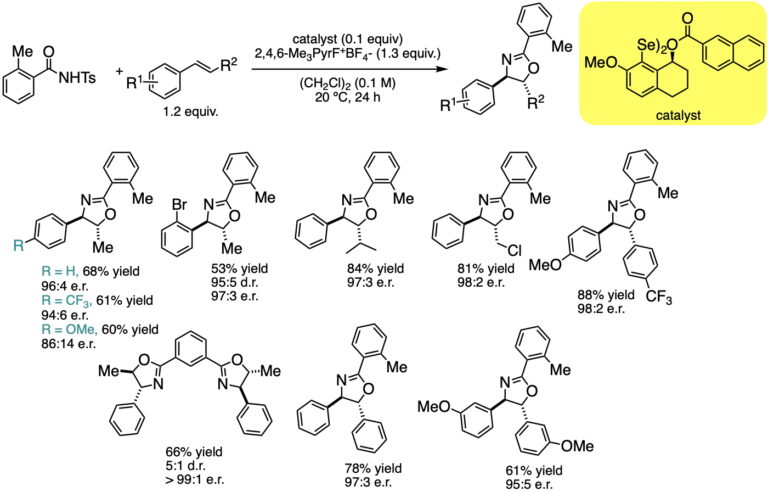
This manifestation of redox catalysis has been expanded to achieve syn-enantioselective oxyamination using N-tosyltoluamides as the bifunctional nucleophiles. The transformation enjoys a similar scope as the diamination, but the initial formation of oxazoles allows for additional downstream transformations.
Catalytic, Enantioselective, Syn-Oxyamination: Scope