Research Overview
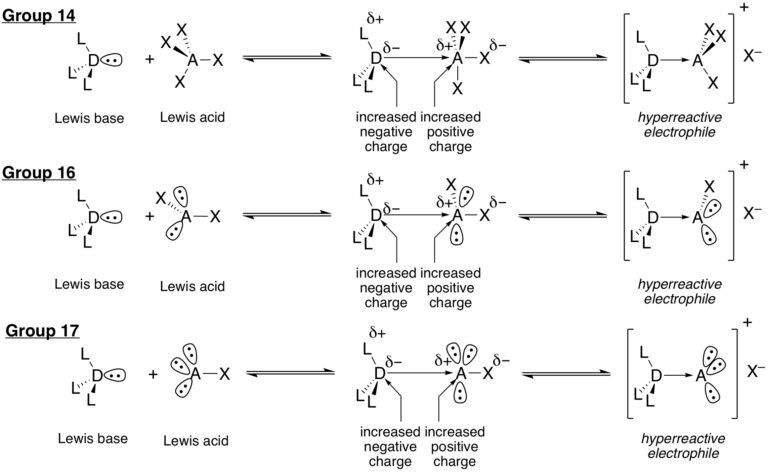
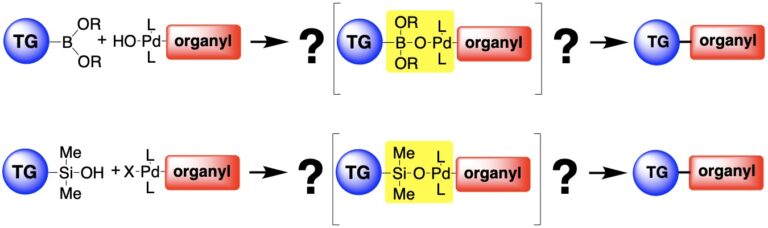
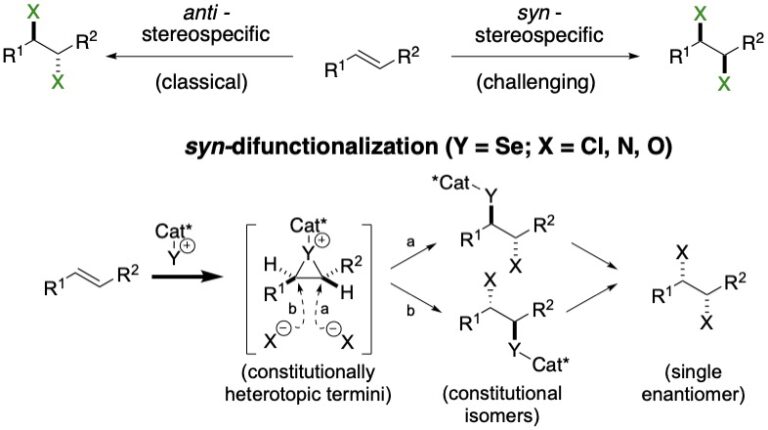
The research in this group is primarily involved with the invention of new synthetic reactions and the origin of stereocontrol in fundamental carbon-carbon bond forming reactions. The long-standing emphasis in our laboratories centers on the relationship between structure, reactivity and stereoselectivity in a variety of organo-element systems such as: palladium-catalyzed, cross-coupling reactions with organofunctional silicon compounds, asymmetric catalysis of carbonyl addition reactions, and applying the reducing power of the Water-Gas Shift Reaction to organic synthesis. We have pioneered the concept of chiral Lewis base activation of Lewis acids for catalysis of electrophilic reactions in the Main Group for elements in Groups 14, 16 and 17. In recent years, we have developed the application of redox catalysis for the stereocontrolled vicinal difunctionalization of unactivated alkenes. Catalysts derived from Group 16 elements in various chiral architectures have been featured in these processes. We maintain a long-standing interest in the structural chemistry and reactivity of organosilicon, -lithium, -phosphorus and –zinc compounds.
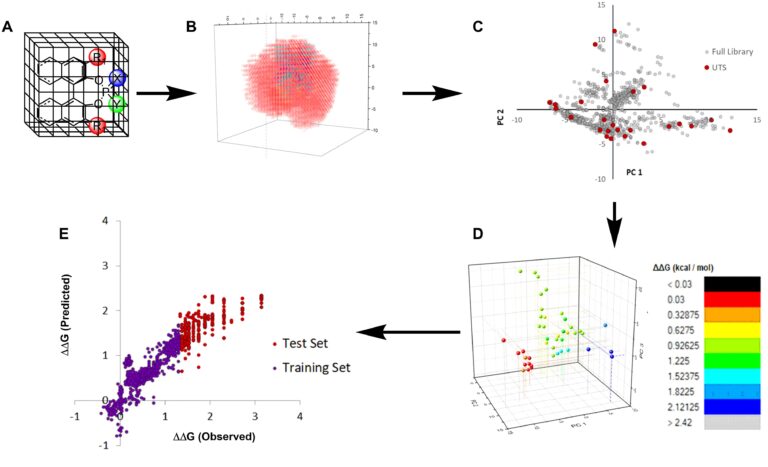
One of the most active and emerging programs is application of artificial intelligence (statistical analysis, machine learning) for two major objectives: (1) the identification and optimization of catalysts for enantioselective reactions, and (2) recommending reaction conditions and making yield predictions for workhorse reactions in organic synthesis. We have introduced fully chemoinformatic-guided workflows for both of these objectives and have experimentally validated the selection of chiral catalysts (from several platforms) and the predictions of yields.