Read the most recent review: 351, 366, 368, 376, 382
The WGSR is of extraordinary importance in maximizing the production of hydrogen from coal gasification and steam reforming. The equilibrium is favorable in both the gas and liquid phases. In electrochemical terms the WGSR has approximately the reduction potential of gallium(0). The primary goal of this project is to engineer catalytic processes for known, synthetically useful carbon-carbon bond forming reactions that couple the reducing power of the WGSR with the generation of reactive organometallic intermediates.
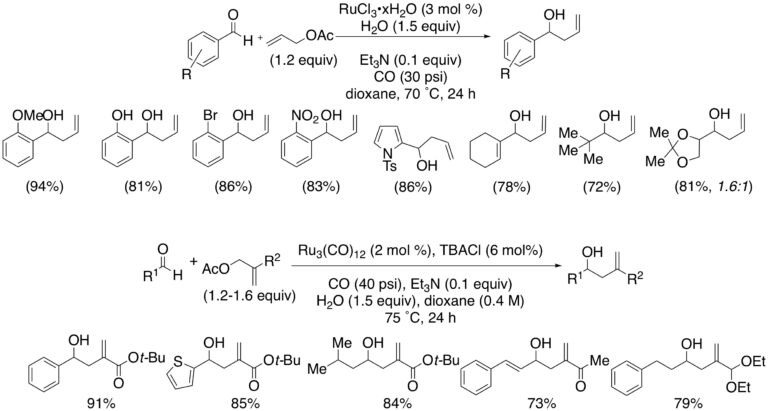
We have developed first example of a ruthenium-catalyzed allylation of aldehydes operating under WGSR conditions. An early report form Watanabe described the formation of homoallylic alcohols under catalysis by ruthenium and using a trialkylamine as the reducing agent under a CO atmosphere. The discovery that the addition of 1.5 equiv of water allowed both the reaction temperature and CO pressure to be significantly decreased enabled the development of a superior process. The reducing potential is provided by the combination of water and CO, namely through the agency of the water-gas shift reaction. The ability of this transformation to accommodate substituents at the 2-position of the allylating reagent has been demonstrated Aryl, alkyl, ester, ketone and acetal functions were all tolerated with aromatic, olefinic and aliphatic aldehydes.
Catalytic Allylation of Aldehydes under WGSR Conditions: Scope:
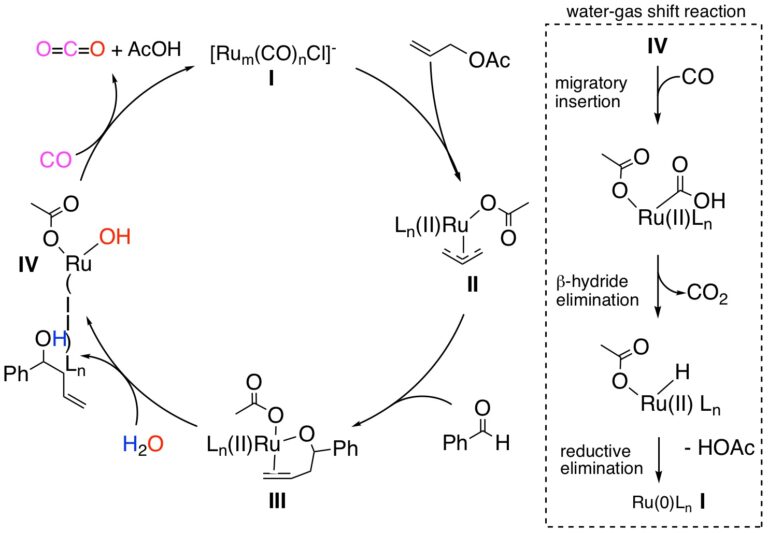
A catalytic cycle for this reaction has been formulated that involves initial reduction ruthenium(III) to the ruthenium(0) species (I) by CO, oxidative addition (OA) to allyl acetate occurs to afford the ruthenium(II) π-allyl species (II). This nucleophilic species (II) next inserts into the aldehyde via coordination of the carbonyl group to the ruthenium(II) center (via the η1 form of I) to generate (III). Hydrolysis of the alkoxide (III) then releases the homoallylic alcohol product and generates a ruthenium(II) hydroxide species (IV). By means of the WGSR, CO then undergoes a migratory insertion into the ruthenium(II)-OH bond, followed by β-hydride elimination to release CO2 (as shown in the inset). Subsequent reductive elimination of the ruthenium(II) hydride intermediate regenerates the ruthenium(0) complex (I).
Catalytic Allylation of Aldehydes under WGSR Conditions: Catalytic Cycle:
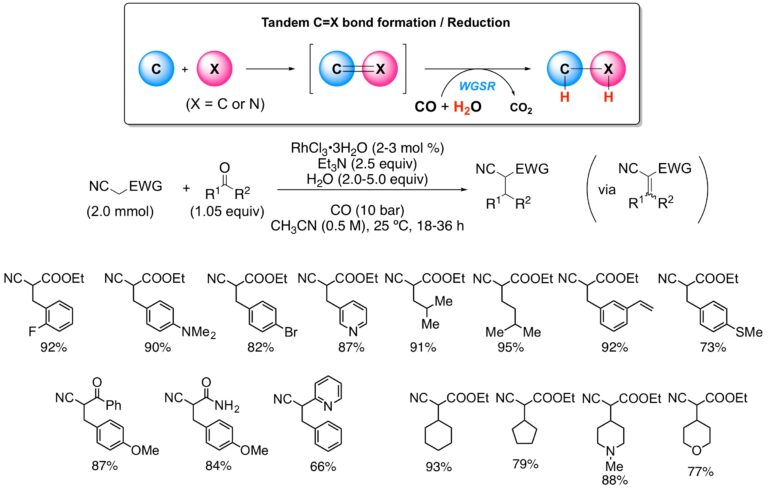
A second preparative application of the rhodium-catalyzed water-gas shift reaction involves the reductive alkylation of several classes of activated methylene compounds. Under catalysis by rhodium trichloride, carbon monoxide, water, and triethylamine, the scope has been successfully expanded to cover a wide range of alkylating agents, including aliphatic and aromatic aldehydes, as well as cyclic ketones, in moderate to high yields. The process combines the in-situ Knovenagel condensation of active methylene compounds with aldehydes and ketones followed by the ready reduction of the newly formed double bond. This method is comparable to, and for certain aspects, surpasses the established reductive alkylation protocols.
Reductive Alkylation of Activated Methylene Compounds under WGSR Conditions:
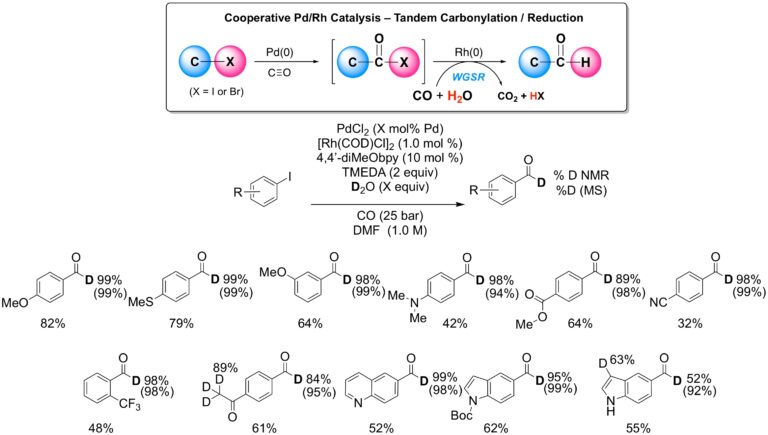
Most recently, a novel, palladium-rhodium, dual-metallic, cooperative catalytic process has been developed to affect the reductive carbonylation of aryl halides in moderate to good yield. In this reaction water is the hydride source and CO serves both as the carbonyl source and the terminal reductant through the Water-Gas Shift Reaction. The catalytic generation of the Rh hydride allows for the selective formation of highly hindered aryl aldehydes that are inaccessible through previously reported reductive carbonylation protocols. Moreover, efficient and selective synthesis of D-formyl aldehydes can be achieved using D2O as a cost-effective deuterium source without the need for pre-synthesizing the aldehyde.
Reductive Carbonylation-Deuteration of Aryl Halides under WGSR Conditions: