Read the most recent reviews and papers: 361, 365, 369, 380, 395, 413
It is axiomatic that the development of new, selective and general carbon-carbon bond forming reactions is central to the mission of organic synthesis. Over the past 30 years, the transition metal catalyzed cross-coupling of organometallic donors with organic acceptors has revolutionized the construction of bonds between unsaturated centers. The impact of this process across the entire chemical landscape is breathtaking and reflected in the number of publications (>7700) appearing on this topic since its formal birth in 1972. Further, the doubling in publication frequency from 1996 (285) to 2000 (554) and again to 2004 (1105) provides compelling testimony to the vigor and outlook for the field.
Perhaps the most dramatic affirmation of the impact of cross-coupling reactions in synthesis is the awarding of the 2010 Nobel Prize in Chemistry to Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki for the development of palladium catalyzed carbon-carbon bond formation.
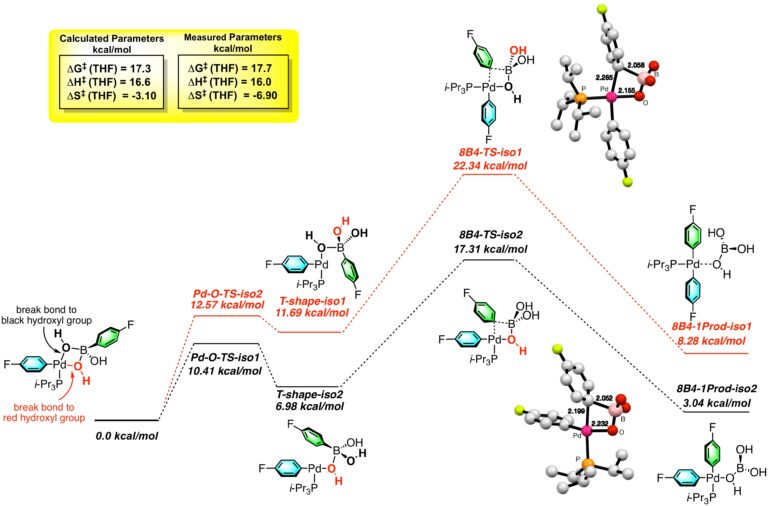
The Suzuki-Miyaura cross-coupling reaction is widely recognized as the most useful and broadly applicable of the various palladium-catalyzed cross-coupling reactions. Surprisingly, the key mechanistic details associated with the critical transmetalation step have remained elusive until our recently reported identification of the “Missing Link”. Using our home-built Rapid-Injection NMR apparatus, we have successfully identified and structurally and kinetically characterized three different intermediates containing the critical B–O–Pd linkage that has been hypothesized but until now never seen. These mechanistic investigations have been bolstered by an extensive computational analysis of the transmetalation pathway using density functional theory. The activation energy of transmetalation transition state for migration of the aryl group in the 8-B-4 intermediate T-Shape-iso-2 is 5.0 kcal/mol lower than from T-Shape-iso-1 because of less steric crowding and also because of bond formation trans to the less donating substituent, the phosphine ligand. The computationally derived activation parameters match with remarkable agreement with those determined by Arrhenius analysis experimentally.
Identification of the Pre-Transmetalation Intermediate in the Suzuki-Miyaura Cross-Coupling Reaction:
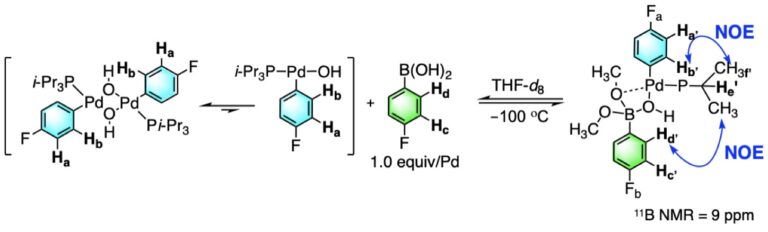
Computational Analysis of the Transmetalation Pathway in the Suzuki-Miyaura Cross-Coupling Reactions:
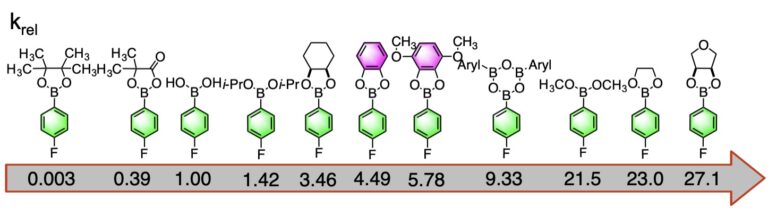
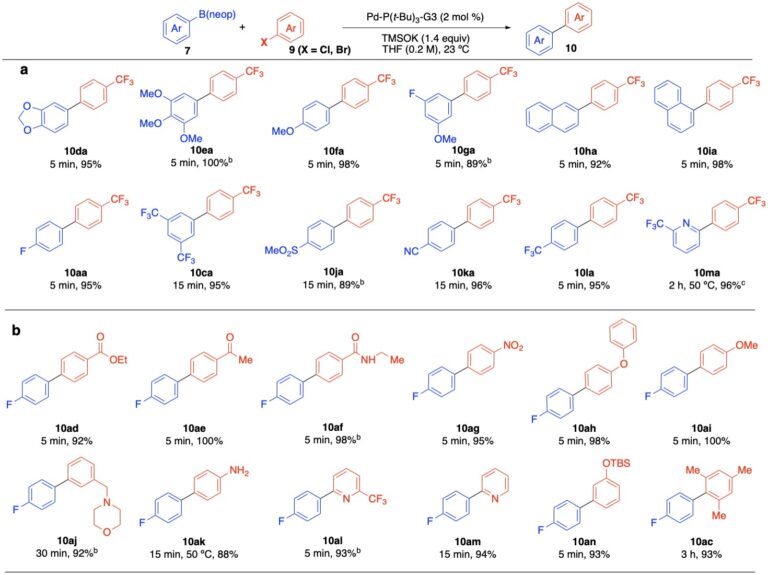
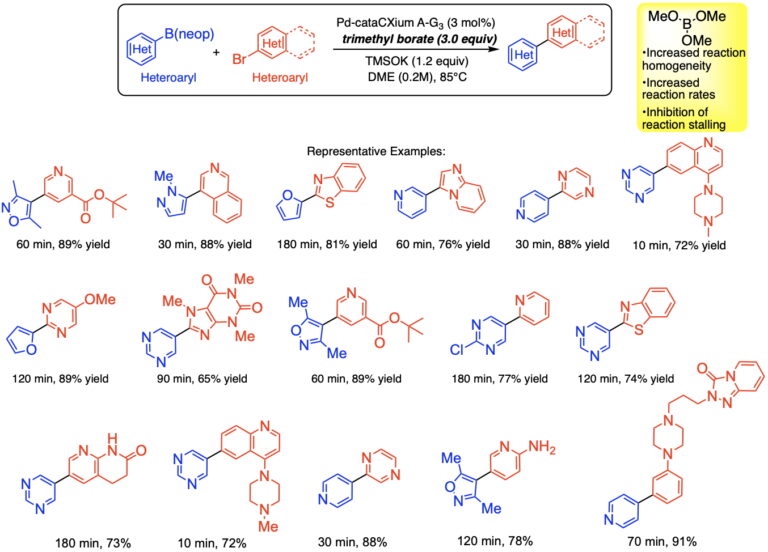
More recent studies reveal the unexpected advantage of carrying out the cross-coupling reaction on the boronic esters under anhydrous conditions to accelerate transmetalation and avoid protodeboronation.
Comparison of Transmetalation Rates of Various Arylboronic Esters under Anhydrous Conditions:
These mechanistic observations led to the development of a new modification of the classical Suzuki-Miyaura cross-coupling conditions in which boronic esters are employed under anhydrous, homogeneous conditions to give rapid reactions devoid of protiodeboronation.
Preparative Examples of Cross-Coupling Arylboronic Esters under Anhydrous Conditions:
In earlier investigations, we discovered that organosilanols are viable partners in palladium-catalyzed cross-coupling reactions under activation by both fluoride and non-fluoride reagents. This discovery has opened a new dimension for the application of silicon-based, carbon-carbon bond forming processes. This research program features advances in the methodological extension of this reaction including the construction of silanol substrates, the scope of basic activators, and the diversification of transferable groups (TG) attached to silicon. A number of powerful constructive processes have been parlayed with cross-coupling to create versatile transformations (hydrosilylation, silylformylation, ring closing metathesis, enyne metathesis, silylcarbocyclization, dipolar cycloaddition). We have engineered sets of reaction conditions that allow for cross-coupling in many structural settings which were heretofore not amenable to palladium catalyzed bond forming processes. We have dramatically expanded the scope of substrates (alkenyl, alkynyl, aryl, heteroaryl) that engage in the coupling.
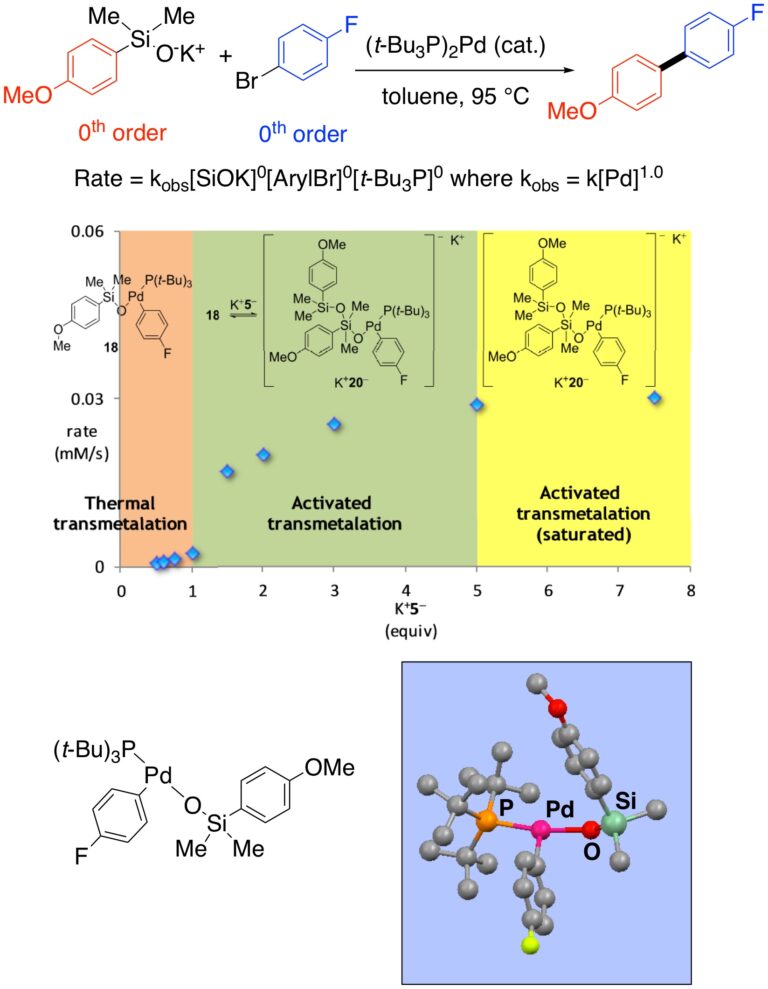
We have carried out extensive mechanistic studies including, F-19, Si-29 NMR, kinetic analysis, natural abundance isotope effect and crystallographic studies and have confirmed the operation of two pathways involving neutral four-coordinate and anionic 5-coordinate silicon species. Kinetic analysis and X-ray crystallographic studies have proven the intermediacy of the critical Si-O-Pd linkage.
Preparative Examples of Cross-Coupling Heterocyclic Arylboronic Esters with Heterocyclic Halides under Anhydrous Conditions with Trimethyl Borate:
In earlier investigations, we discovered that organosilanols are viable partners in palladium-catalyzed cross-coupling reactions under activation by both fluoride and non-fluoride reagents. This discovery has opened a new dimension for the application of silicon-based, carbon-carbon bond forming processes. This research program features advances in the methodological extension of this reaction including the construction of silanol substrates, the scope of basic activators, and the diversification of transferable groups (TG) attached to silicon. A number of powerful constructive processes have been parlayed with cross-coupling to create versatile transformations (hydrosilylation, silylformylation, ring closing metathesis, enyne metathesis, silylcarbocyclization, dipolar cycloaddition). We have engineered sets of reaction conditions that allow for cross-coupling in many structural settings which were heretofore not amenable to palladium catalyzed bond forming processes. We have dramatically expanded the scope of substrates (alkenyl, alkynyl, aryl, heteroaryl) that engage in the coupling.
Cross-Coupling Reactions of Organosilanols and Silanolates:
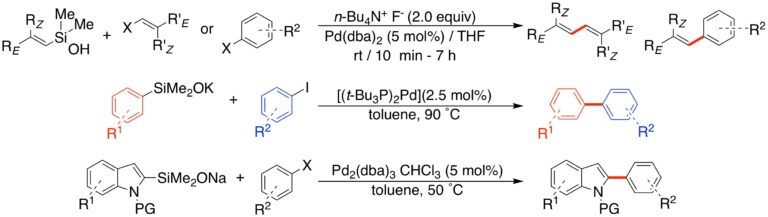
A particularly important development is the introduction of alkali metal silanolate salts. These compounds are stable, free-flowing powders that are self-activating (require no base or fluoride) and are compatible with a wide range of functional groups.
We have carried out extensive mechanistic studies including, F-19, Si-29 NMR, kinetic analysis, natural abundance isotope effect and crystallographic studies and have confirmed the operation of two pathways involving neutral four-coordinate and anionic 5-coordinate silicon species. Kinetic analysis and X-ray crystallographic studies have proven the intermediacy of the critical Si-O-Pd linkage.
Kinetics and Mechanism of Silicon-Based Cross-Coupling Reactions:
The synthetic utility of the silicon-based, cross-coupling process has been highlighted in the total syntheses of a 9-membered ring ether, (+)-brasilenyne, a C-aryl glycoside, papulacandin D, two kaianic acid analogs, isodomoic acids G and H, and the polyene chain in RK-397.
Total Synthesis with Silicon-Based Cross-Coupling Reactions: