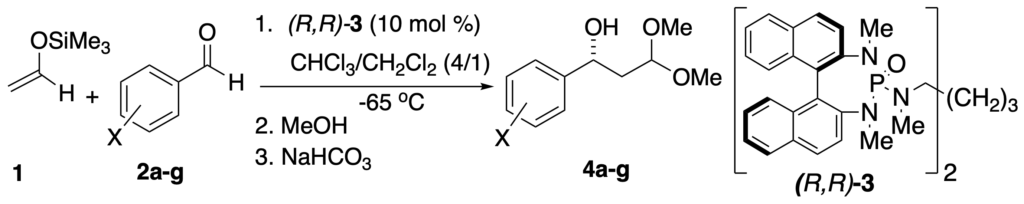
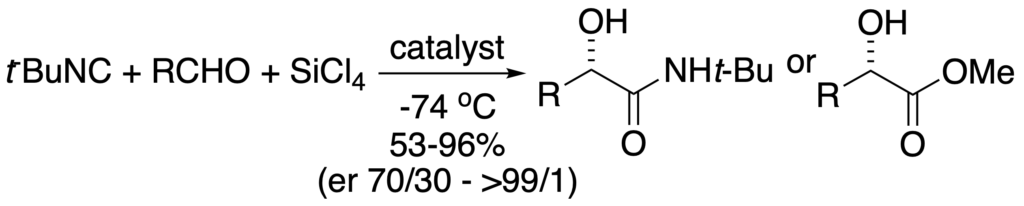
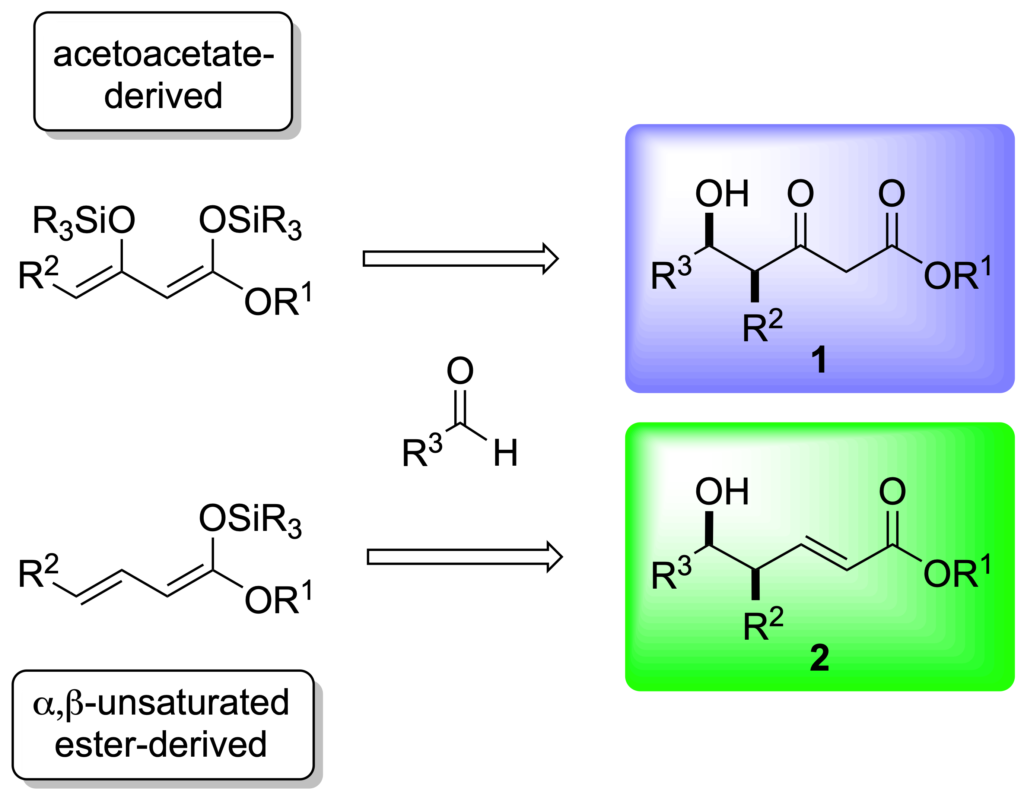
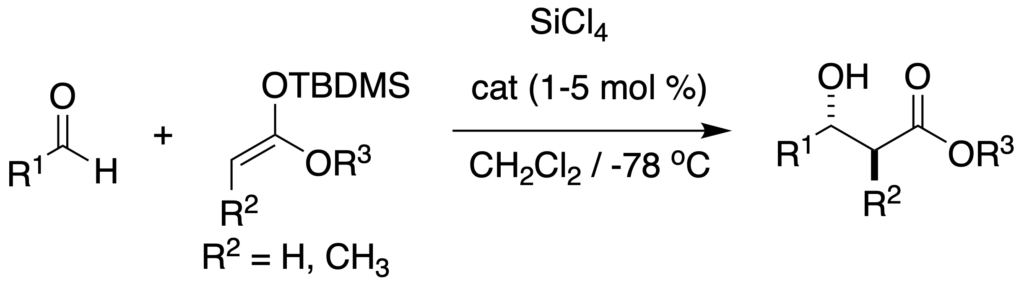[342] Lewis Base Catalysis of the Mukaiyama Directed Aldol Reaction: 40 Years of Inspiration and Advances (with G. L. Beutner) Angew. Chem. Int. Ed. 2013, 52, 9086-9096.
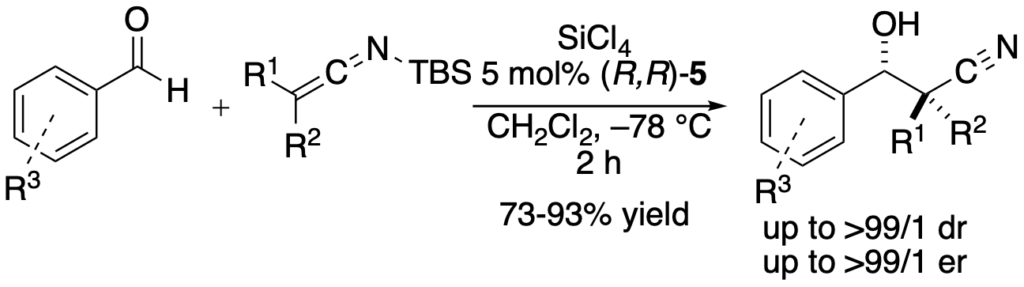
[337] Silyl Ketene Imines: Highly Versatile Nucleophiles for Catalytic, Asymmetric Synthesis (with T. W. Wilson) Angew. Chem. Int. Ed. 2012, 51, 9980-9992.
[334] Lewis Base Catalyzed Enantioselective Additions of an N-Silyl Vinylketene Imine (with T. W. Wilson) Angew. Chem. Int. Ed. 2012, 51, 3236-3239.
[321] N-Silyl Oxyketene Imines are Underused Yet Highly Versatile Reagents for Catalytic Asymmetric Synthesis (with T. W. Wilson) Nature Chemistry 2010, 2, 937-943.
[307] On the Mechanism of Lewis Base Catalyzed Aldol Addition Reactions: Kinetic and Spectroscopic Investigations Using Rapid-Injection NMR (with B. M. Eklov, P. J. Yao, and M. D. Eastgate) J. Am. Chem. Soc. 2009, 131, 11770-11787.
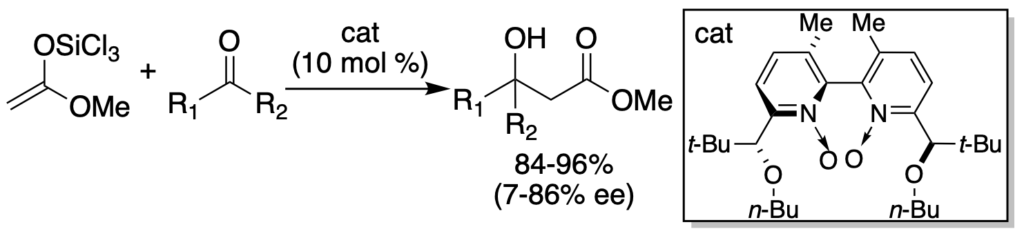
[293] Lewis Base Activation of Lewis Acids: Catalytic, Enantioselective Addition of Glycolate-Derived Silyl Ketene Acetals to Aldehydes (with W. Chung) J. Org. Chem. 2008, 73, 4582-4595.
[291] Methyl Trichlorosilyl Ketene Acetal (with Y. Fan) e-EROS Encyclopedia of Reagents for Organic Synthesis [Online] 2008.
[286] Lewis Base Activation of Lewis Acids: Catalytic Enantioselective Glycolate Aldol Reactions (with Won-jin Chung) Angew. Chem. Int. Ed. 2008, 47, 1890-1892.
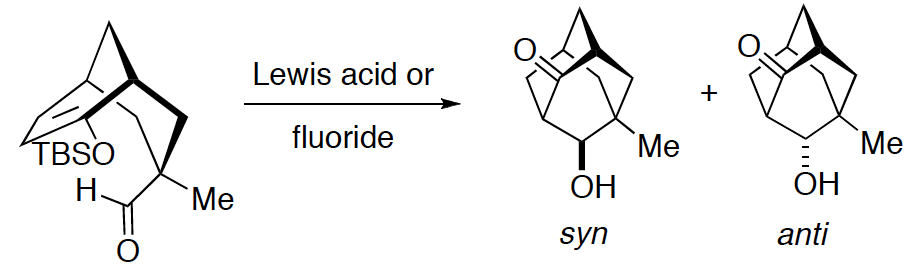
[283] Investigations into Transition-State Geometry in the Mukaiyama Directed Aldol Reaction ( with W. Lee) Chem. Asian J. 2008, 327-341.
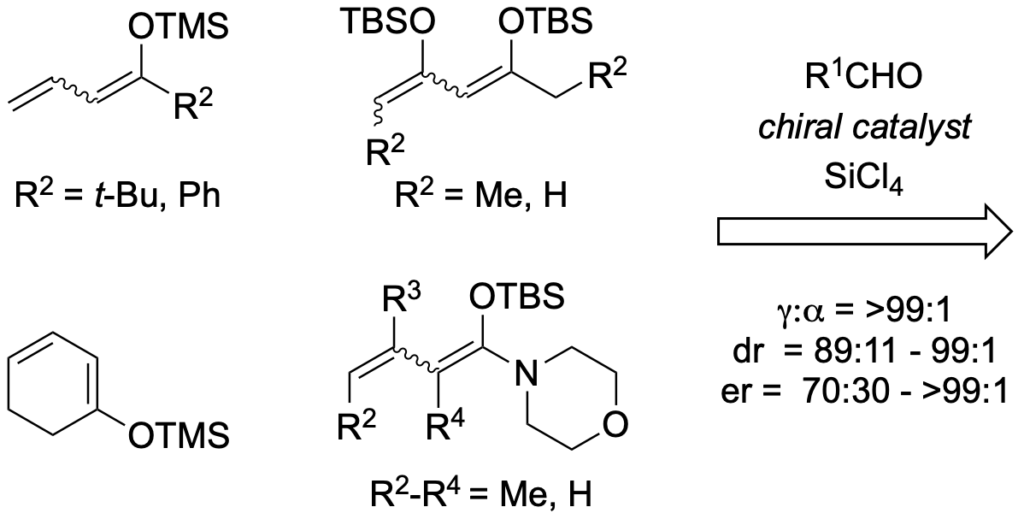
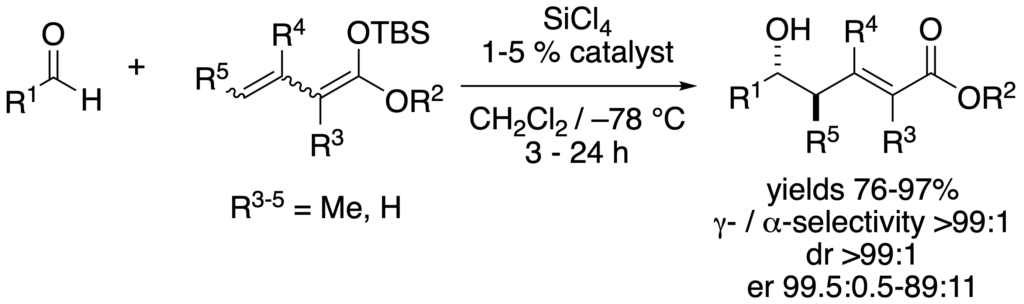
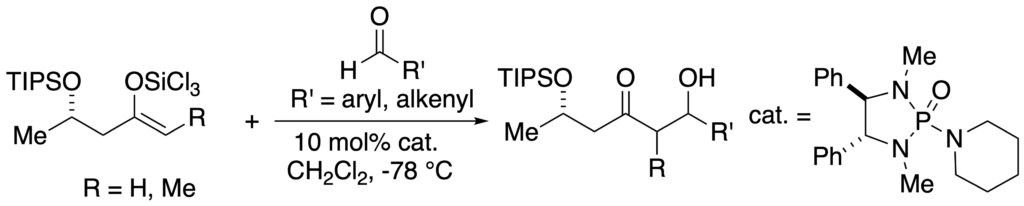
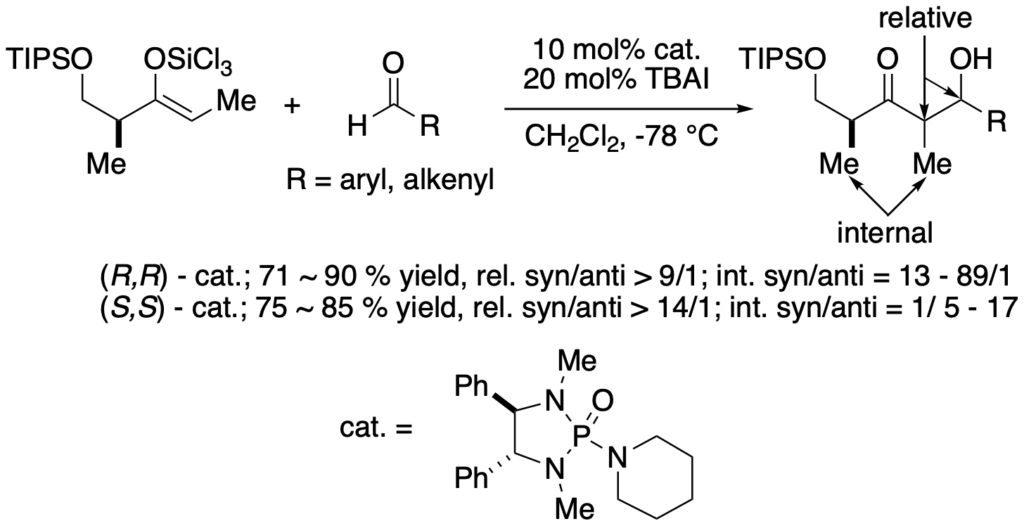
[275] Lewis Base Activation of Lewis Acids: Catalytic, Enantioselective Vinylogous Aldol Addition Reactions (with J. R. Heemstra) J. Org. Chem. 2007, 72, 5668-5688.
[274] Unexpected Ambidoselectivity in Crossed-Aldol Reaction of a-Oxy Aldehyde Trichlorosilyl Enolates (with S. K. Ghosh) Tetrahedron Prize Special Issue 2007, 63, 8636-8644.
[254] Lewis Base Activation of Lewis Acids. Vinylogous Aldol Addition Reactions of Conjugated N,O-Silyl Ketene Acetals to Aldehydes (with J. R. Heemstra, Jr.) J. Am. Chem. Soc. 2006, 128, 1038-1039.
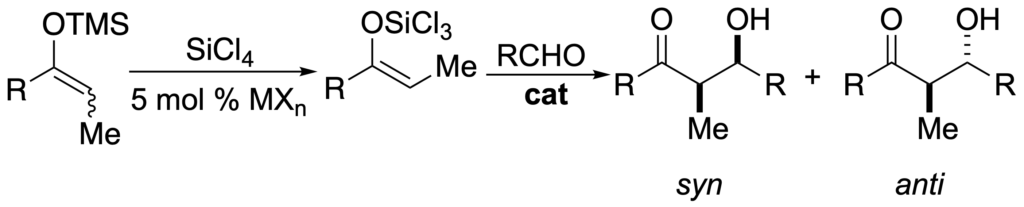
[250] Lewis Base Catalyzed Aldol Additions of Chiral Trichlorosilyl Enolates and Silyl Enol Ethers (with J. Fujimori and S. M. Pham) J. Org. Chem. 2005, 70, 10823-10840.
[249] Mechanistic Insights into the Chiral Phosphoramide-Catalyzed, Enantioselective Crossed-Aldol Reactions of Aldehydes (with T. Bui) J. Org. Chem. 2005, 70, 10393-10397.
[247] Lewis Base-Catalyzed Enantioselective Aldol Addition of Acetaldehyde-Derived Silyl Enol Ether to Aldehydes (with T. Bui) J. Org. Chem. 2005, 70, 10190-10193.
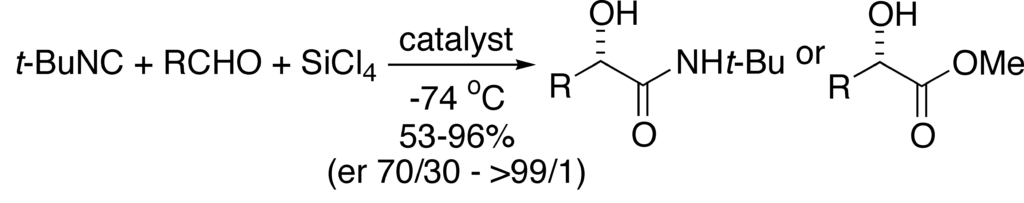
[246] Catalytic, Enantioselective alpha-Additions of Isocyanides: Lewis-Base-Catalyzed Passerini-Type Reactions (with Y. Fan) J. Org. Chem. 2005, 70, 9667-9676.
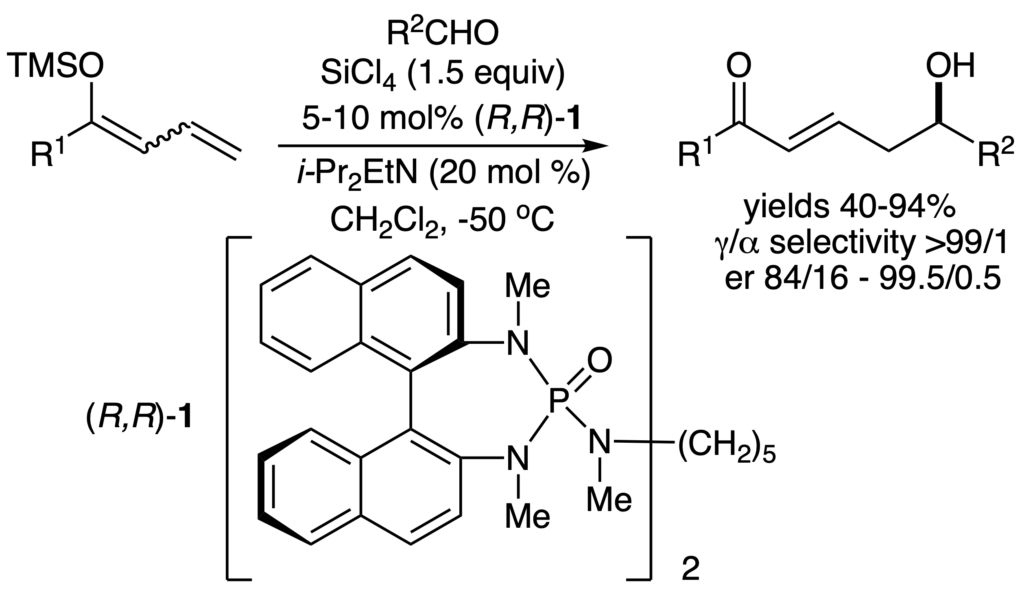
[245] Catalytic, Enantioselective, Vinylogous Aldol Reactions (with J. R. Heemstra, Jr. and G. L. Beutner) Angew. Chem. Int. Ed. 2005, 44, 4682-4698.
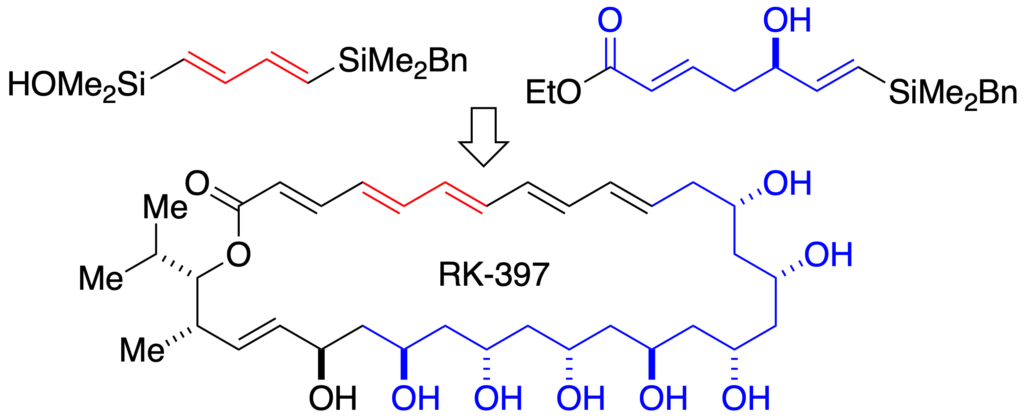
[244] Total Synthesis of RK-397 (with S. Fujimori) J. Am. Chem. Soc. 2005, 127, 8971-8973.
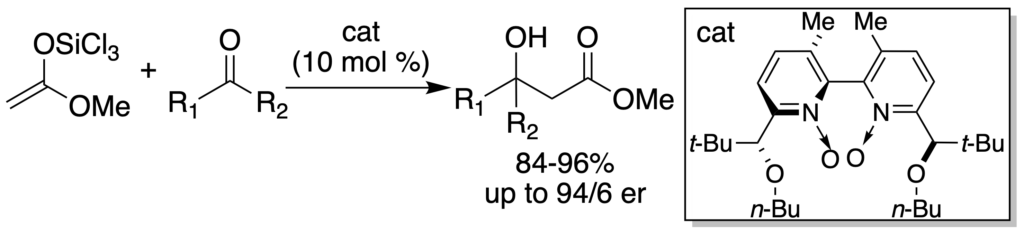
[243] Lewis Base Catalyzed, Enantioselective Aldol Addition of Methyl Trichlorosilyl Ketene Acetal to Ketones (with Y. Fan and M. D. Eastgate) J. Org. Chem. 2005, 70, 5235-5248.
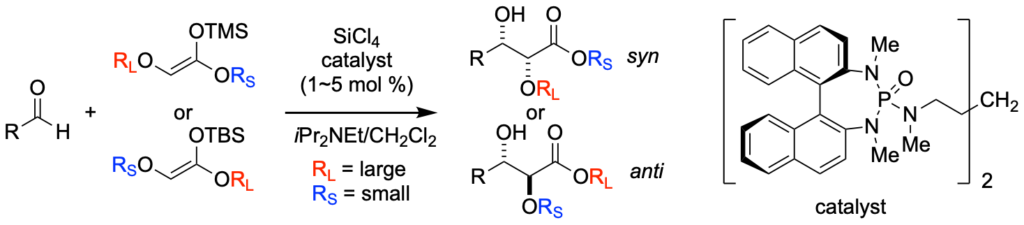
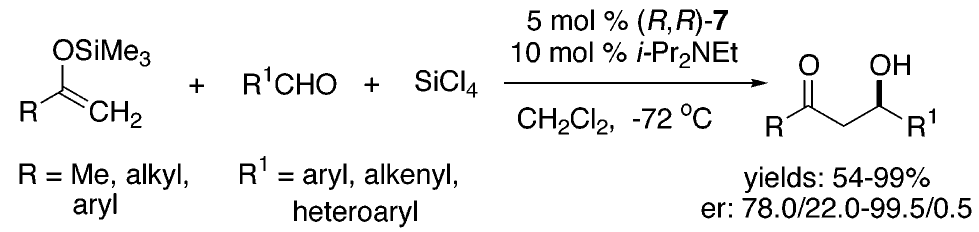
[239] Lewis Base Activiation of Lewis Acids: Catalytic, Enantioselective Addition of Silyl Ketene Acetals to Aldehydes (with G. L. Beutner, T. Wynn, and M. D. Eastgate) J. AM. Chem. Soc. 2005, 127, 3774-3789.
[235] Lewis Base Activation of Lewis Acids: Vinylogous Aldol Additions of Dienol Ethers to Aldehydes (with J. R. Heemstra Jr.) Synlett 2004, 13, 2411-2416.
[227] Chiral Phosphoramide-Catalyzed, Enantioselective, Directed Cross-Aldol Reactions of Aldehydes (with T. Bui) Proc. Nat. Acad. Sci. 2004, 101, 5439-5444.
[219] The First Catalytic, Asymmetric alpha -Additions of Isocyanides. Lewis-Base-Catalyzed, Enantioselective Passerini-Type Reactions (with Y. Fan) J. Am. Chem. Soc. 2003, 125, 7825-7827.
[218] Lewis Base Activation of Lewis Acids. Vinylogous Aldol Reactions (with G. L. Beutner) J. Am. Chem. Soc. 2003, 125, 7800-7801.
[216] Stereoselective Aldol Additions of Achiral Ethyl Ketone-Derived Trichlorosilyl Enolates (with S. M. Pham) J. Org. Chem. 2003, 68, 5045-5055.
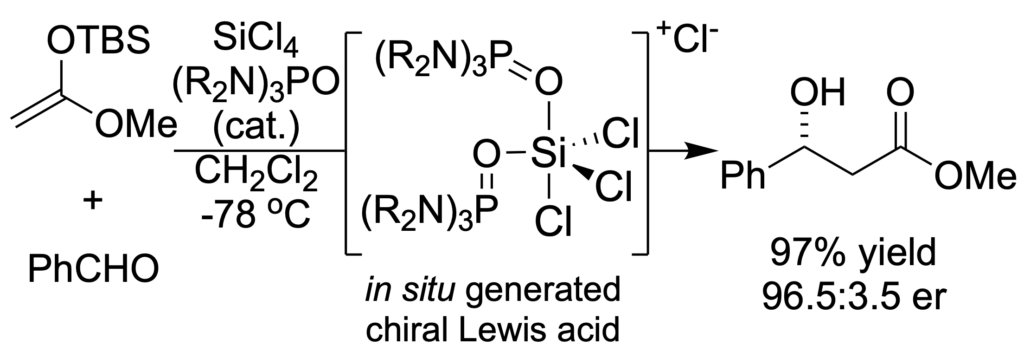
[205] Lewis Base Activation of Lewis Acids. Addition of Silyl Ketene Acetals to Aldehydes (with T. Wynn and G. L. Beutner) J. Am. Chem. Soc. 2002, 124, 13405-13407.
[201] The Effects of a Remote Stereogenic Center in the Lewis Base Catalyzed Aldol Additions of Chiral Trichlorosilyl Enolates (with S. Fujimori) Org. Lett. 2002, 4, 3477-3480.
[200] Diastereoselective Aldol Additions of Chiral beta -Hydroxy Ethyl Ketone Enolates Catalyzed by Lewis Bases (with S. Fujimori) Org. Lett. 2002, 4, 3473-3476.
[196] Catalytic, Enantioselective Aldol Additions to Ketones (with Y. Fan) J. Am. Chem. Soc. 2002, 124, 4233-4235.
[192] The First Catalytic, Diastereoselective, and Enantioselective Crossed-Aldol Reactions of Aldehydes (with S. K. Ghosh) Angew. Chem. Int. Ed. 2001, 40, 4759-4762.
[189] Highly Diastereoselective Aldol Additions of a Chiral Ethyl Ketone Enolate Under Lewis Base Catalysis (with S. M. Pham) Org. Lett. 2001, 3, 2201-2204.
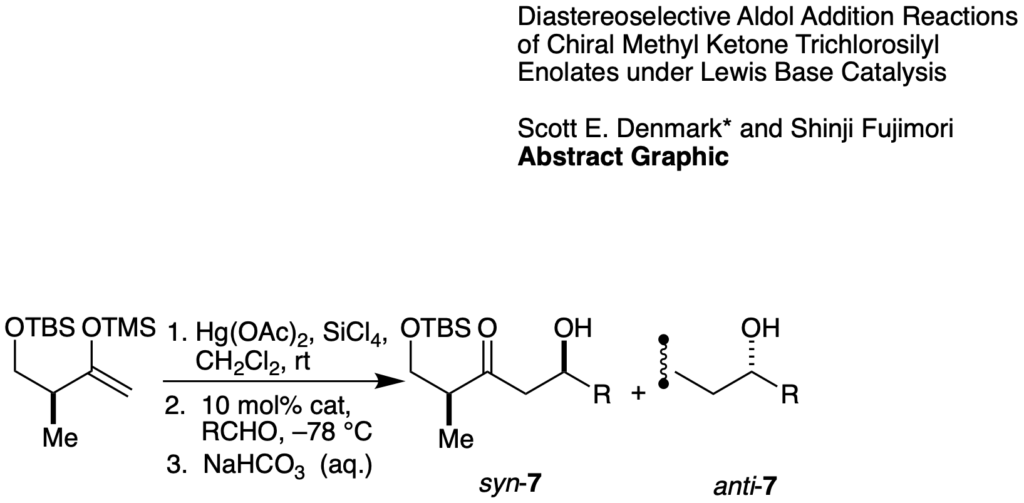
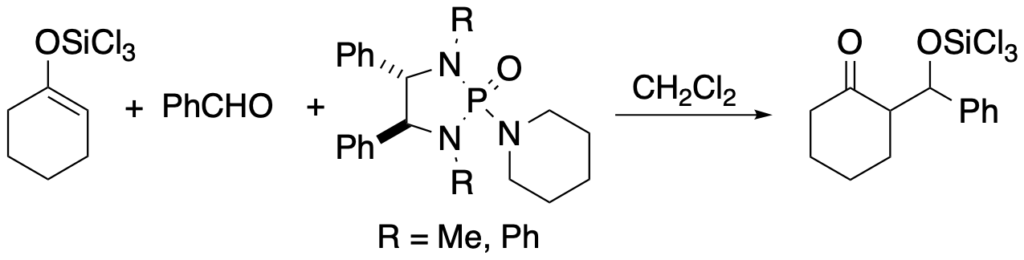
[188] Diastereoselective Aldol Addition Reactions of a Chiral Methyl Ketone Trichlorosilyl Enolate under Lewis Base Catalysis (with S. Fujimori) Synlett 2001, SI, 1024-1029.
[177] The Chemistry of Trichlorosilyl Enolates. Aldol Addition Reactions of Methyl Ketones (with R. A. Stavenger) J. Am. Chem. Soc. 2000, 122, 8837-8847.
[171] Asymmetric Catalysis of Aldol Reactions with Chiral Lewis Bases (with R. A. Stavenger) Accounts Chem. Res. 2000, 33, 432-440.
[164] Solid State and Solution Structural Studies of Chiral Phosphoramide-Tin Complexes Relevant to Lewis Base Catalyzed Aldol Addition Reactions (with X. Su) Tetrahedron 1999, 55, 8727-8738.
[162] Chiral Phosphoramide-Catalyzed Aldol Additions of Ketone Enolates. Preparative Aspects (with R. A. Stavenger, K.-T. Wong and X. Su) J. Am. Chem. Soc. 1999, 121, 4982-4991.
[157] The Chemistry of Trichlorosilyl Enolates. 6. Mechanistic Duality in the Lewis Base-Catalyzed Aldol Addition Reaction (with X. Su and Y. Nishigaichi) J. Am. Chem.Soc. 1998, 120, 12990-12991.
[156] Highly 1,4-syn Diastereoselective, Phosphoramide-Catalyzed Aldol Additions of Chiral Methyl Ketone Enolates (with R. A. Stavenger) J. Org. Chem. 1998, 63, 9524-9527.
[155] Preparation of Chlorosilyl Enolates (with R. A. Stavenger, S. B. D. Winter, K.-T. Wong and P. A. Barsanti) J. Org. Chem. 1998, 63, 9517-9523.
[154] Asymmetric Catalysis with Chiral Lewis Bases (with R. A. Stavenger, X. Su, K.-T. Wong and Y. Nishigaichi) Pure Appl. Chem. 1998, 70, 1469-1476.
[151] Asymmetric Aldol Additions Catalyzed by Chiral Phosphoramides: Electronic Effects of the Aldehyde Component (with R. A. Stavenger and K.-T. Wong) Tetrahedron 1998, 54, 10389-10402.
[143] Lewis Base-Catalyzed, Asymmetric Aldol Additions of Methyl Ketone Enolates (with R. A. Stavenger and K.-T. Wong) J. Org. Chem. 1998, 63, 918-919.
[132] The Chemistry of Trichlorosilyl Enolates. 2. Highly-Selective Asymmetric Aldol Additions of Ketone Enolates (with K.-T. Wong and R. A. Stavenger) J. Am. Chem. Soc. 1997, 119, 2333-2334.
[120] The Chemistry of Trichlorosilyl Enolates. 1. New Reagents for Catalytic Asymmetric Aldol Additions (with S. B. D. Winter, X. Su and K.-T. Wong) J. Am. Chem. Soc. 1996, 118, 7404-7405.
[97] The Chemistry of Enoxysilacyclobutanes. 3. Uncatalyzed, Syn-Selective, Asymmetric Aldol Additions (with B. D. Griedel) J. Org. Chem. 1994, 59, 5136-5138.
[93] The Chemistry of Enoxysilacyclobutanes: Highly Selective Uncatalyzed Aldol Additions (with B. D. Griedel, D. M. Coe and M. E. Schnute) J. Am. Chem. Soc. 1994, 116, 7026-7043.
[92] Triarylcarbenium Ions as Catalysts in the Mukaiyama Aldol Addition: A Mechanistic Investigation (with C.-T. Chen) Tetrahedron Lett. 1994, 35, 4327-4330.
[89] Investigations on Transition-State Geometry in the Lewis Acid- (Mukaiyama) and Fluoride-Promoted Aldol Reaction (with W. Lee) J. Org. Chem. 1994, 59, 707-709.
[79] The Chemistry of Enoxysilacyclobutanes: Highly Selective, Uncatalyzed Aldol Additions (with B. D. Griedel and D. M. Coe) J. Org. Chem. 1993, 58, 988-990.
[75] Investigations on Transition State Geometry in the Aldol Condensation in Aqueous Medium (with W. Lee) Tetrahedron Lett. 1992, 33, 7729-7732.
[59] Investigations on Transition State Geometry in the Aldol Condensation (with B. R. Henke) J. Am. Chem. Soc. 1991, 113, 2177-2194.
[41] Investigations on Transition State Geometry in the Aldol Condensation (with B. R. Henke) J. Am. Chem. Soc. 1989, 111, 8032-8034.
- Group 16 and 17 Lewis Base Catalysis
- Syn-Difunctionalization of Alkenes
- Chemoinformatics
- Boron-Based Cross-Coupling Reactions
- Silicon-Based Cross-Coupling Reactions
- Water-Gas Shift Reaction
- Phase Transfer Catalysis
- Organoelement Chemistry
- Allylmetal Aldehyde and Acetal Reactions
- Aldol Chemistry
- Nitroalkene and Related Cycloaddition (Including Tandem)
- Chemistry of Phosphorus-Stabilized Anions
- Dioxirane Chemistry
- Silicon-Directed Nazarov Cyclization and Related Reactions
- Carbanion-Accelerated Claisen Rearrangements
- Perspectives and Reviews
- Miscellaneous